What is Clean-in-place (CIP)?

Making food and pharmaceutical processing safe and efficient is a primary goal for all processing plants, and cleaning in place plays an integral part of this ecosystem. Clean-in-place (CIP) systems designed specifically for the food, dairy, beverage, and pharmaceutical processing industries deliver a faster and repeatable approach to avoiding risks to product quality and integrity.
For processors, the steps of purchasing and installing a CIP system can be a considerable undertaking, requiring extensive analysis, planning, and cost to complete. This article is designed as a general overview of CIP to help readers understand the basics and next steps.
This resource will outline
- The basics of CIP
- Industries that utilize CIP
- The anatomy of a CIP system
Let's dive in!

Basics of CIP
Cleaning in place (CIP) is a set of activities conducted to properly clean all or part of a process system as it sits in place, without removing or disassembling piping or equipment to accommodate the cleaning.
CIP systems distribute
- Cleaning solutions
- Rinsing solutions
- Sanitizing solutions
These solutions are run through the same piping path as the product to eliminate product soil from all internal surfaces.
Depending on the system and the product being cleaned your CIP system could be as simple as a mobile CIP cart to clean one small circuit or as complex as a multi-tank, multi-supply system equipped to service multiple circuits or kitchens simultaneously.
Note: Cleaning procedures must be carried out safely due to the harsh chemicals involved that can be harmful to people and equipment. A well-designed CIP system virtually eliminates operator exposure to chemicals.
Cleaning in place should always be conducted with the least possible impact on the environment by minimizing water and detergent usage and by maximizing the recovery and re-use of resources.
Industries that Utilize CIP
CIP systems vary widely in
- Configuration
- Capacity
- Quality
- Level of automation
They also vary by industry. Differences in product characteristics and regulatory considerations between the various processing industries strongly impact the design of your CIP system.
Food, Dairy, and Beverage
The number of CIP applications for food, dairy, and beverage process systems has grown dramatically in recent years. Processors in these industries are recognizing the importance of fast, efficient clean in place.
The range of product types and cleaning requirements in these industries is virtually endless, so when you are looking to install or upgrade a CIP system it’s important that you consider your particular cleaning needs.
A “cookie cutter” solution that purports to sufficiently clean any application might not necessarily be the proper solution for your system.
- The surface finish for product contact areas tends to be the standard sanitary surface finish of 32Ra minimum. This is rougher than the finishes required in the pharmaceutical world, but it is smooth enough to allow most product residues to release fairly easily.
- The CIP cycles may include pre-wash, various detergent/acid wash cycles, and rinse cycles. They may also include a sterilization cycle for an extended shelf life (ESL) product. Sterilization is also common for pharmaceutical CIP.
- CIP applications require less time and energy and fewer chemicals when the product is less viscous and doesn’t contain proteins or fats. The acidity of some products also naturally inhibits the growth of microorganisms, which could mean having to clean less frequently.
- Milk and dairy products are particularly vulnerable to spoilage and the rapid growth of bacteria. Therefore CIP systems in dairies must be very specific regarding temperatures, cycle times, and chemical concentrations to effectively prevent contamination from harmful microorganisms such as salmonella, listeria and E. coli.




Breweries
Most modern breweries are highly automated closed systems, so they lend themselves well to cleaning in place.
The vessels used in brewing sometimes have a large capacity and may require multiple spray devices to be properly cleaned. A phosphoric acid wash may be required in some cases to remove beerstone buildup.

Pharmaceutical
The highest level of CIP technology and state-of-the-art controls are usually found in pharmaceutical systems. The exacting standards of the industry demand a level of hygiene, documentation, and automation that isn’t required in other processing industries.
- Process line sizes tend to be smaller and the product is often less viscous than many food products, so high flow volumes and powerful spray devices may not be necessary for proper cleaning. The design of pharmaceutical process piping should be sloped properly and be free of undrainable low points.
Anatomy of A CIP System
Although many food, dairy, beverage, and pharmaceutical processors go about the business of making their products in similar ways, one thing is certain: every system is unique.
There is no such thing as “one-size-fits-all” when it comes to CIP systems. Although all CIP systems may vary, several system elements are common in most systems. Here is a look at them.
Controls, Instrumentation, & Automation
CIP systems must meet and maintain critical parameters for the duration of the cleaning cycle. If the specification is not met, cleaning must be repeated.
Manual CIP is labor intensive. It requires an operator to physically oversee the CIP system as it runs, leaving room for human error, which ultimately leads to inconsistent cycles with improper or incomplete cleaning. With the Food Safety Modernization Act (FSMA), consistency, accuracy, and repeatability are more important than ever.
Automated CIP calculates, tracks, and runs CIP independently, allowing the operator freedom to focus elsewhere while maintaining repeatability and consistency of the cleaning process.

There are other benefits to utilizing automation.
1. Repeatability: With manual CIP, each operator is tasked to perform cleaning based on a set of written procedures dictated by the plant. Many times cleaning steps are done poorly or not done at all. Automated CIP provides a repeatable, consistent process that does not rely on human performance by automatically controlling pump speeds, chemical concentrations, and temperatures through a computer to ensure the same results every time. If any of the parameters are not met during the cycle, personnel can see where the deviations occur and address them.
2. Accurate data recording: For each individual CIP sequence, temperature, flow, concentration, and cycle times are recorded. Data recording allows users to validate the system was cleaned.
3. Reduced utility consumption: Conserving resources, like water, electricity, and steam is becoming increasingly important in modern businesses. High-efficiency pumps and plate and frame heat exchangers (like the Alfa Laval LKH and TS6/M10) allow for reduced electrical and steam usage. Additionally, automated, centralized CIP can allow the recovery and reuse of water during the CIP process. Utility conservation is an important key to cutting costs.
Documentation
In addition to controlling and monitoring your CIP cycles, a suitable control system should also provide documentation for batch records to verify cleaning as part of a validation and verification process. Some control systems can store records onboard for easy retrieval.
The control panel, or Human Machine Interface (HMI) must be simple and intuitive with user-friendly graphics so the operator can understand the status of the cleaning operation “at a glance.” A touchscreen option makes viewing access even quicker and easier by eliminating the need for a separate keyboard.
The controls should be able to build and store multiple programs for various circuits while also allowing changes to wash cycles in real time. The controls system should have Ethernet communication capabilities to allow it to interface with process control systems for a truly integrated cleaning solution.
Tanks
The size and number of tanks in your CIP system is determined by the size and complexity of required cleaning.
- Individual tanks may be used for freshwater, caustic solution, acid solution, reusable washes or rinses, rinse recovery or sanitizer.
- Tank design depends primarily on the industry, but the materials of construction are commonly austenitic stainless steels such as AISI 304, 316 or 316L.
- Using 304 stainless steel for some food, dairy, beverage, and brewery CIP tank applications are acceptable, but most quality CIP system manufacturers feature 316/316L as the standard material for all wetted components, including tanks.
A cleaning system may use one to four tanks, or more, depending on the application. Although no one formula exists for system design, some common considerations apply for most applications.

Single-Tank System
- Ideal for small jobs with easily cleaned soil.
- Good for special use systems that are infrequently used.
- Ideal for applications on remote equipment that make service from a large, centralized system impractical.
- Often preferred for avoiding allergens and cross-contamination issues since each cycle is dumped to the drain after a single pass.
- Requires less space and can easily be cart-mounted for mobility.
Two-Tank System
- Typically one tank is for water (for pre-rinse or final rinse) and one for caustic wash solution.
- Can easily be cart-mounted for mobility.
Three-Tank System
- Allows for many more cleaning options than smaller systems.
- Water/caustic/acid: Common for many cleaning applications. Allows for pre-fill and pre-heat of all solutions, which reduces cleaning time. Caustic and acid washes can be returned and re-used, depending on the amount of soil.
Four-Tank System
- The additional tank provides the flexibility to recover and re-use solutions without sacrificing the convenience of individual ready-to-go caustic and acid solutions.
Few CIP systems require more than four tanks, but additional tanks can be added to any system as necessary to provide nearly unlimited versatility and convenience to the CIP cleaning process.
Depending on the type of cleaning needed, each tank must be fitted with all necessary inlet and outlet valves as well as any instrumentation required to monitor volume, temperature, and chemical concentrations.
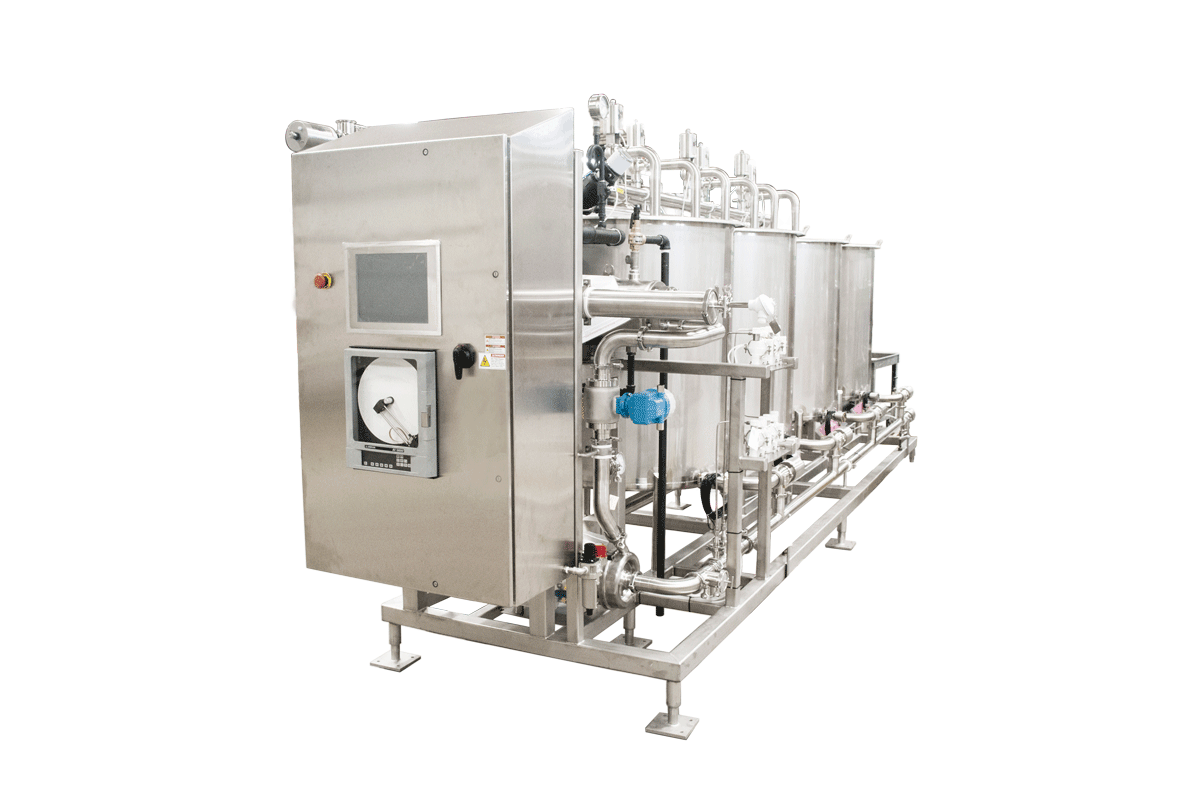
Pumps
CIP systems rely on pumps to provide the proper flow and pressure of CIP solutions to the process areas. Process engineers take various factors into consideration such as length of CIP circuits and types of process equipment to be cleaned to properly size and specify CIP supply pumps.
In addition to the supply pumps on the CIP system, other pumps are required to provide effective CIP within the facility. These pumps can include return pumps, chemical addition pumps, and sometimes CIP boost pumps.
Heating Systems
Temperature is one of the four basic TACT principles essential to the cleaning process, so choosing the right heating source for your CIP system is an important decision.
Elevating the temperature of your cleaning solutions will increase their soil-removing efficiency, which in turn will reduce your cleaning time and chemical costs.
Each CIP heating system has its own advantages. When choosing, consider the available plant resources.
- Plate & Frame Heat Exchanger – These units are highly efficient and typically have a small footprint allowing a more compact design of the CIP system. Additionally, they have a relatively low steam load requirement, making them a desirable choice for CIP systems.
- Shell & Tube Heat Exchanger – A simple, low-maintenance in-line heating option. While these units are widely used in the food, dairy, and beverage markets, they are typically found in pharmaceutical applications where complete drainability is required.
- Direct Steam Injection – These units inject precise amounts of steam directly into the cleaning solution as it is being pumped to the process.
- Electric Heater – Although sometimes slower and potentially more costly to operate than other options, electric heaters can be a prudent choice if other heating options are unavailable.

Piping and Valves
The network of piping and valves in a CIP system is designed to allow proper control of each process step with maximum efficiency.
Piping
- Should be the proper diameter to provide flow and pressure requirements
- Should take the shortest route possible
- Should be free from dead legs
Valves
They “control the traffic” in a system. They can operate manually or automatically, but manual valves require operator intervention for each step of the cleaning cycle.
- A number of different valves can be used depending on industry, application and amount of automation needed. Your CIP designer may choose from seat, butterfly, or diaphragm valves.
Next Steps
Purchasing and installing a CIP system for your processing facility is a considerable undertaking, requiring analysis, planning and above all, partners.
The best “first step” you can take is to create a team of knowledgeable operators, managers from multiple departments, and most importantly a trusted company that has vast experience in designing and building process and CIP systems.
That’s where we come in.
CSI engineers, designs, and fabricates custom clean-in-place systems to meet specific hygienic processing needs. CIP equipment from CSI helps you control, monitor, and document the cleaning methods that are essential to sanitary processing.
With CSI's state-of-the-art, climate-controlled fabrication shop, the quality of equipment leaving our facility is second to none. We offer in-house, Level II inspection in accordance with the latest ASNT recommended Practice No. SNT-TC-1A, so you can be certain your equipment meets industry standards.
To speak with a CIP expert request a quote below or call 800.654.5635.
Clean-in-place Buying Guide
This Buying Guide for Clean-in-Place Solutions is a comprehensive resource for anyone who designs, owns, or operates processing systems and wants information about all aspects of CIP Systems.
ABOUT CSI
Central States Industrial Equipment (CSI) is a leader in distribution of hygienic pipe, valves, fittings, pumps, heat exchangers, and MRO supplies for hygienic industrial processors, with four distribution facilities across the U.S. CSI also provides detail design and execution for hygienic process systems in the food, dairy, beverage, pharmaceutical, biotechnology, and personal care industries. Specializing in process piping, system start-ups, and cleaning systems, CSI leverages technology, intellectual property, and industry expertise to deliver solutions to processing problems. More information can be found at www.csidesigns.com.


